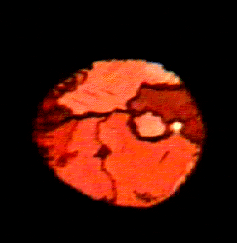
Red oxygen crystal. Photo from the
American Institute of Physics.
Red Oxygen is more than just an international SMS provider and an awesome Australian band; it’s what happens to oxygen (O2) molecules when submitted to pressures above 10 GPa. Once under pressure, the O2 molecules condense to a pale blue liquid, then a pale blue solid and finally a red solid.
In 2006, physicist Malcolm McMahon at the University of Edinburgh studied the structure of the red oxygen crystal by surrounding it in helium. They discovered that in this state, the four O2 molecules act like a squashed cube and, unpredictably, formed a single O8 molecule that did not move into a ring shape. This changed the way scientists understood the behavior of dense oxygen. The study was only possible due to advances in atomic and high-pressure technology.
Currently, there are no astrophysical settings where red oxygen is prevalent. Scientists believe that if the structures could be retained at normal pressures, the crystals could make an excellent rocket fuel.

